Asymmetrical and Dust Charging
Electrical phenomena associated with sand and dust storms, blizzards and
falling snow have been reported for centuries. Explosions from coal dusts in
mines, flour, chocolate powder and grain dust in silos pose hazards. And in
some volcanic eruptions lightning in dust clouds, that is, in largely
non-phreatic ejectamenta has received some investigation.
The electrification of what I will collectively call dust clouds was
noticed in the 18th century when the study of static electricity
became a primary interest. Amongst the
investigations of the Rev Abraham Bennet, using his new invention of the gold
leaf electrometer was what happened when a small pile of dust placed on the cap
of the electrometer was blown away. The
leaves diverged.
Thales, Gilbert,
On the hypothetical side various charging mechanisms were proposed to
account for how friction between materials produced a separation of electrical
charges.
In the early twentieth century Frictional Electrification was given the
name Tribo-electrification. ‘A rose by any other name would smell as sweet’ W. Shakespeare A peculiarity of human nature. If you cannot
explain it –change its name.
Throughout this Electro-magnetic Miscellany I have for the most part
deliberately excluded Mathematics. This
qualitative approach is to give you both the results of some experimentation
and to help you either repeat experiments, devise your
own OR take up an investigation to take further something which has taken your
fancy.
I will now précis one series of static electrification experiments made
in the early twentieth century by Professor Peter. S. Shaw of
One basic notion which came from the goodness knows how many frictional
experiments over two or more centuries was that unlike materials were needed to
produce positively and negatively charged objects; glass and silk, ebonite and
fur, nylon (carpet) and shoe leather,
chocolate and tooth enamel and on and on.
Attempts were made to form a Tribo-electric series. To as it were
parallel static electrification of various materials in a similar manner to the
series of metals in electro-chemical cells.
This was in part successful but the temperamental behaviour of
electrostatics brought, and brings, in controversy as to which materials should
be placed where in the series i.e. in the list.
Peter Shaw set out to make yet another attempt at a Tribo-electric
series and discovered something of basic importance. Namely that like materials rubbed in a
particular way will also generate unlike polarities of electrical charge. (It may be that this had been seen earlier
but Shaw made an intensive study of the phenomena.)
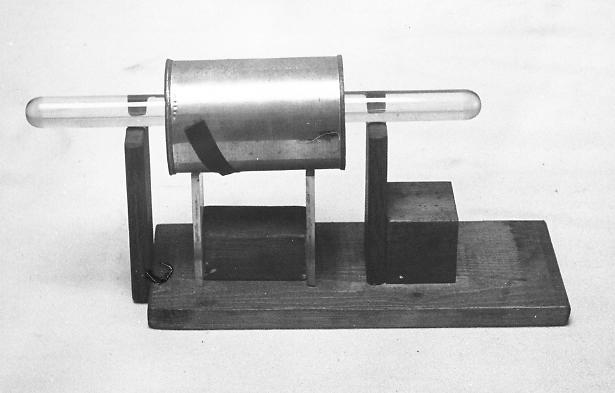
This is my version of Shaw’s apparatus. A conductor/ tin with ends
removed and smoothed, rests on two vertical Perspex insulators. A charged sample – in this case illustrated
by a glass rod – is placed inside but not touching the conductive
cylinder. The cylinder is connected to
an electrometer – (in my work –it was a modern Kelvin Quadrant Electrometer
made by WPA Instruments of Cambridge, England).
The whole of the above is placed, when in use, inside a Faraday cage to
prevent extraneous charges from outside affecting the experiment.
Shaw investigated glass, ebonite and ice as his main materials. I used Polystyrene, Perspex and glass.
It is important to prepare clean material from the same sample, in the
form of two rods or bars.
One piece is rubbed asymmetrically on the other.
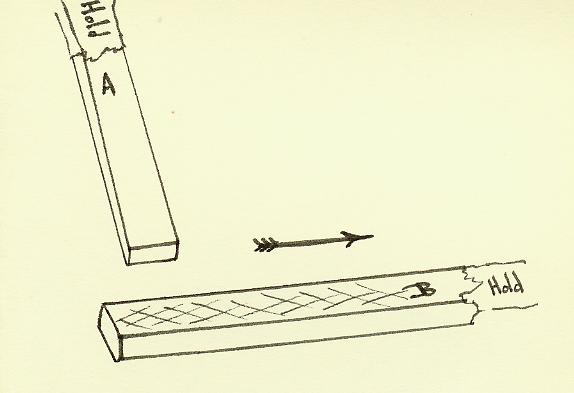
Dia A
Hold a rod in each hand.
Rub a small area of Rod A along most of the length of Rod B. Do this several times. Carefully place a rod inside the conducting
cylinder and note polarity. Do this
again with the other rod.
The polarity on one rod is
opposite to that on the other.
REMEMBER this is electrostatics! Most of the time things work but often,
when you least expect it, they do not; usually when you are demonstrating an
effect to someone else as I have just theoretically done for you.
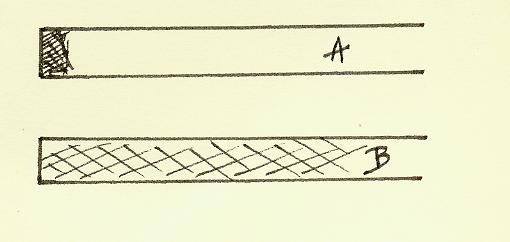
Dia B
If you examine the surfaces which have been in contact wear is
apparent. I found glass to be very
temperamental, due I suspect to humidity.
Shaw prepared his glass samples meticulously as you may read in his
paper. Evenso
he had his moments as we might say!
I dare say that this experiment would, within error of measurement
limitations, demonstrate that equal but opposite charges exist on the rods.
What is important is the fact that:-
Like Materials will, when in
asymmetric contact, generate unlike electrical polarities of charge.
P.E Shaw & C.S.Jex. Tribo-electricity
and friction. Proc. Roy. Soc.A Vol 3,758.1926
P.E.Shaw. Experiments on tribo-electricity. 1. The tribo-electric
series. Vol
94, 656,
Nov.1917.
P.E.Shaw & C.S.Jex.Tribo-electricity and friction.II. Glass & Solid
Elements. Vol 118, 779, March 1928.
As above III. Solid Elements &
Textiles. Vol.118, 779, March 1928.
P.E.Shaw. The Electrical
Charges from Like Solids. Nature, Letters, Nov.6 1926.
P.E.Shaw. Electrical Separation Between
Identical Solid Surfaces. Physics & Physical Soc. Papers.
Dust
P.E.Shaw. Tribo-electricity
and friction.IV. Electricity due to Air Blown
Particles. Proc.Roy. Soc. A. Vol 122. 789.
W.A.
Douglas Rudge. On the Electrification Produced during the
Raising of a Cloud of Dust. Proc. Roy. Soc. Vol. 90. 618.
I will mention just these two papers but there must be very many more by
now. Back in the 1970s Kamra was investigating Indian Dust Storms and Mills used
dry sand in an evacuated flask to demonstrate the possible source of lightning
on Mars.
A reasonably simple set up for investigating dust electrification is
this one:-

This was my initial apparatus – shown without its Faraday cage.
The horizontal copper tube is 2 inches diameter, which gives you an idea
of the scale. The small copper funnel, on the left, was in a stand at the left
hand end of the tube. Air was supplied
by a reversed vacuum cleaner. A measured
weight of powder was poured into the funnel and blown along the pipe into the
large vertical cylinder. Air left at the
top and dust settled to the bottom which could be on an earthed metal plate or
a sheet of insulator.
Various experiments were tried.
For like on like material the whole apparatus was lined with paper first
coated with the kind of powder to be used.
For simple trials it was used as shown here.
Some kinds of powder used.
Graphite Aluminium Zinc Copper Titanium
dioxide
Silicon Silicon dioxide Sand Pumice
Dust from Vesuvius Coal dust Flour Silicon carbide
The basic powders were ANALAR from a chemical supply company, others were collected in the field.
( Be very wary of metal powders
and flour they can ignite. Wear a mask
to prevent inhalation.)
In the first trials the simple leaf electrometer worked to show that it
was worth all the effort! Later and
using very small quantities of dust a quadrant electrometer was used in place
of the simple leaf electrometer.
Using like on like
demonstrated that in practice asymmetrical charging occurred and in consequence
of which if a dust cloud were to be
constituted of only one kind of powder it will charge with electricity.