
Leidenfrost`s Phenomenon
J.G.Leidenfrost
`Thou shalt separate the earth from the fire`
Hermes Trismegistus

The Wonderful Doctor of Duisburg - Johann Gotlobb Leidenfrost (1715 - 1794)
Johann Gottlob Leidenfrost was born in Ortenberg in the County of Stollberg Germany on the 24th of November 1715. His father Johann Heinrich Leidenfrost was a vicar and intended that his son should study Theology. However Johann Gottlob found his interest was more in medicine and he studied in Geissen, Leipzig and Halle where, in 1741, he was awarded his Doctorate in Medicine, M.D., for a highly acclaimed thesis -`On the Harmonious Relationship of Movements in the Human Body`.
On his travels he met many patrons and had discussions with King Frederich. During the Silesian War Leidenfrost was a field doctor. On the 14th September 1743 he was appointed to a medical professorship at the University of Duisburg on the Rhine. Despite many tempting offers from other universities Professor Doctor Leidenfrost remained at Duisburg.
He was admired as a lecturer who could fire interest in the driest subjects and for practical demonstrations. His interests ranged over Mathematics and Physics, History, Pedagogics and Philosophy. In addition to his University work he had a GP practice which attracted patients from outside Duisburg. His medical correspondence demanded several hours a day and he slept only a few hours each night.
His name is immortalised in his investigation of what is now named the Leidenfrost Phenomenon, an aspect of boiling which has to be taken into account in such diverse fields as Cryogenics, Astrophysics & Cosmology, Mass Spectroscopy and the temperature control of electronic devices.
The Prussian kings expressed their goodwill and he was appointed into membership of the Berlin Academy of Science.
The 50 years of his jubilee as Professor was celebrated by the striking of a medal bearing his portrait. A shy man he spent the festive day alone in silence.
He was a real Christian, calm and resigned whose wife (nee Kaldhoff ) had died some years earlier, when his end came on the 2nd of December 1794.
Countless students who had sent their own sons to be taught by him made him a monument.
The complete list of his writings on diverse subjects was included in the `Geneological Table of the Leidenfrost Family` by his descendant Dr. Robert Leidenfrost, vicar in Graz, in 1876. His biography, `On the Life, Character and Merit of J.G. Leidenfrost` was published by Dr.A.W. Moller in Duisburg in 1795.

Published in 1756 in the University of Duisburg
(A Tract About Some Qualities of Common Water)
This is the extremely rare Leidenfrost Tractatus of which Chapter 15:
![]()
(On the Fixation of Water in Diverse Fire)
deals with the Leidenfrost Phenomenon.
`An iron spoon of any size, well polished within and free from rust and dirt, is heated over glowing coals until it glows with light. To this glowing spoon, removed from the coals, send through a glass tube of suitable length, of which the other end finishes in a very narrow capillary canal, one drop of very pure distilled water... such a tube as I have just described is right to use so that one drop always equal to another falls from the small opening, nor does varying in the magnitude of drops make any difference in the experiment.
This drop which first fell upon the glowing iron is divided into a few little globes, which nevertheless after a little while are collected in one great globe again. At the instant when the drop touches the glowing iron, it is spherical. It does not adhere to the spoon, as water is accustomed to do, which touches colder iron. Nevertheless in the first moment of contact the glowing iron around the drop is black, indeed very black in a space which is greater, the brighter the iron, as if the matter of light and fire from the glowing iron is suddenly snatched into the water.
2.If then the spoon remains motionless, this water globule will lie quiet and without any visible motion, without any bubbling, very clear like a crystalline globe, always spherical, adhering nowhere to the spoon, but touching it in one point. However, although motion is not visible in the pure drop, nevertheless it delights in a very swift motion of turning, which is seen when a small coloured speck, for example some black carbon, adheres to the drop. For this is turned around the drop with a wonderful velocity...
Moreover, however this drop only evaporates very slowly. For if you turn to a pendulum indicating seconds with its oscillations, at least 34 or 35 seconds, that it runs a little over half a minute of an hour before the whole drop disappears. Which at last exceedingly diminished so that it can hardly any more be seen, with an audible crack, which with the ears one easily hears, it finishes its existence, and in the spoon it leaves a small particle of earth......`
(An extract from the translation by Carolyn Wares © 1966 University of Oklahoma, Norman,Oklahoma.)
Leidenfrost`s observation of the noise when the drop finally disappears, the `particle of earth` and the title of the chapter - On the Fixation of Water... is alchemical. It looks to the conception of the World made of four elements which by various means can be transformed into one another. Fixation was particularly sought after. He dismisses Boerhaave`s suggestion that the dust inevitably blowing around in the chemistry lab gets into the water.
Johann Gottlob Leidenfrost was convinced of the alchemical fixation of water into earth by the use of various fires. This essentially was because he was at a point in time when the still prevalent Aristotlean approach was slowly but surely giving way to experimental exploration in science.
BOILING
Basically there are four forms of boiling:-
1. Nucleate boiling - The one most people are familiar with in making drinks, washing pots and clothes and cooking. When the temperature of water, say in a saucepan, begins to rise small bubbles of dissolved gases are released. As temperature increases bubbles will be seen at the bottom and on the sides near the bottom of the pan. Here a sharp point or protrusion of the pan surface serves as a nucleate point at which the temperature is highest and the water vapourises. The weight of water above holds the tiny bubbles in place.
No matter how well polished a surface may appear an electron miscrograph will reveal in fact the surface is - as someone well described it - full of mesa like protruberences!
Increasing temperature sees more vapour bubbles until finally these are breaking free and the bulk water is boiling with bubbles forming on the pan surfaces and on any nuclear material - dust - pollen grains - in the water.
2. Flash boiling - at a surface temperature higher than that for nucleate boiling , water poured on the surface is vapourised, with much noise, into steam. This form of boiling is used in certain boilers for rapid steam production.
3.Transition boiling - on a surface higher than for flash boiling a water breaks up into many tiny droplets which bounce on the hot surface and vapourise noisily into steam.
4.Film boiling - The Leidenfrost Phenomenon - When a surface is at a much higher temperature than that of boiling water (100 degrees C) water will first contact the surface and lift clear to hover on its own vapour layer. Vapourisation takes minutes as against seconds for lower surface temperatures.
Investigations into the forms of boiling have been unable to establish precise transition points of surface temperature at which one form will change to the next form. For the most part this is due to surface conditions and the thermal conductive properties of the material from which the hot surface is made.
My Investigation of the Leidenfrost Phenomenon
At my first meeting with Professor Bill Bright I was asked to decide on the subject of research for my doctorate. I decided to pursue the solution contact charging source of Volcanic Lightning.
On the train home I pondered on how the transition boiling, which Blanchard had discovered to produce charge, could somehow be as it were slowed down and the droplets magnified. There came into my mind the idea of increasing the temperature of the hot surface and setting up drops of solution several millilitres in volume. Thus the evaporation time would be increased and the behaviour of a drop on the hot surface could be observed. I did not know that this was called Leidenfrost boiling nor that it would turn out to be different to charge generation by transition boiling.
I have titled this My Investigation solely to avoid confusion with all the other investigations going on into Leidenfrost/ film boiling in the late 1960s and 1970s and to separate out my work from theirs - though their publications obviously affected what I thought - whether anything I did influenced anyone else I do not know. I therefore apologise in advance for use of the personal pronoun.
The Leidenfrost Phenomenon was not a new discovery. Anyone who worked at the blacksmith`s forge was aware of it when cooling iron in the trough. The housewife preparing to smooth clothes first spat on the flat iron taken from near the fire to test the temperature. Those who observed lava pouring into the sea saw the strange phenomenon of large drops of water swishing about on molten lava.
Professor John Tyndall succeeded Michael Faraday at the Royal Institution in London, England. In his book Heat A Mode of Motion he included demonstrations of the Leidenfrost Phenomenon.
A few of Tyndalls illustrations will help in explanations and also, as a bonus, give a glimpse of his demonstrations.
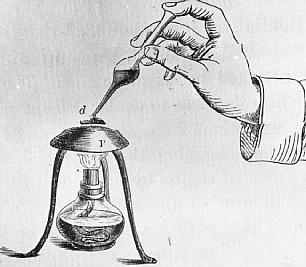
Tyndall`s illustration of the setting up of a Leidenfrost drop
I used shallow stainless steel dishes or a piece of brass about 5 inches (10cm) diameter and 1/2 inch (1cm approximately) in thickness with a shallow depression machined into it (I found it in a scrap bin!). Heat was supplied from a large electric boiling ring which could be adjusted to vary the temperature of the brass dish.

A 1.5 ml drop of distilled water on a surface at 600 degrees C.
Drops were placed from a glass pipette or from hypodermic syringes. On initial contact they hissed very briefly as a series of separate drops rushed around on the hot surface before all coalescing into one drop.
The hot surface causes vapourisation from the underside of the drop. The steam forms a layer which lifts and supports the drop above the hot surface - in the manner of a Hovercraft.
Although this drop, photographed at 1/1000s appears to be stationary, there are convection currents within the water and across its surface. There is rapid movement of vapour up and over the drop. A speck of Balsa wood or a few spores of Lycopodium show this to be the case.
Objectives of my Investigation:-
I set out to study the behaviour of drops - Measure the time drops of different volumes took to evaporate - measure the thickness of the supporting vapour layer and watch for any differences between drops of distilled water and others made from saline solution.

Some drops suddenly change from the drop shape shown to illustrate the 1.5ml. They make this beautiful rosette - here sketched by Tyndall - and some give out a fluttering sound and, more rarely a musical note.

When such a drop is photographed, to record the rosette as this one was, it is disappointing. Examination of many such drops reveal that first of all a circular drop takes on an elliptical shape. Then it becomes an oscillating polygon. Such a polygon is a curvelinear polygon - think of looking end on at the rim of a bell; when the bell is struck the rim oscillates in the form of a curvilinear polygon.
Persistence of vision makes the drop look as Tyndall drew it.
Drops above about 2.5ml in volume describe all manner of shapes on the hot surface and the supporting vapour layer bulges upwards as the dome of a bubble.

The vapour layer lifting the drop centre into a dome.
(White dot is reflected flash- dotted lines are brass surface at 600 deg C)
This does not always occur, then again there may be several such domes in a large drop i.e. up to the 10ml limit I put on my work.

The upper surface of a drop is often covered with wavelike disturbances stopped here at 1/1000s. A circular drop will rarely have circular waves coming from the centre - as if a stone has been dropped into a pool of water - and these flow outwards and disappear under the drop.

(The drop is not on a slope - my photo is!) The differences in drop thickness as it glides on the hot surface. Steam rises,invisibly of course, up and over the drop where some is here condensing and become visible.
The Vapour Layer
I am not including graphs, measurements or calculations - just basic details :
Drops used were at the average salinity 3.5% of the worlds seas and oceans.
I used three approaches to measuring the thickness of the supporting vapour layer. Apart from the very smallest volumes, which take on a nearly spherical shape, drops will have a concave base. The part of the layer of particular interest is at the bottom edge from which steam is escaping and keeping the drop from touching the hot surface. The first approach used photography.
Photography

Tyndall was able to see the light of a glowing filament (a-b left) through the vapour layer beneath the suspended drop. This implied a photographic attempt should be possible.
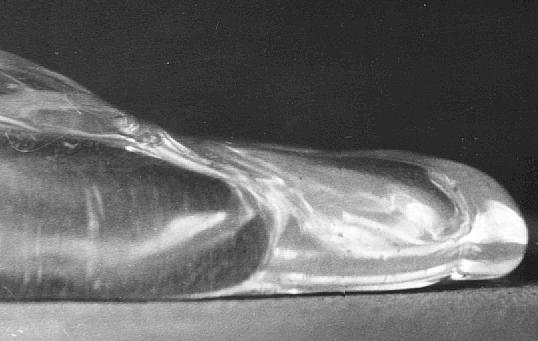
Part of an enlarged photograph of a Leidenfrost drop.
Photographs were taken of drops and enlarged. A scale placed vertically on the brass surface was photographed. Direct measurements were made at equal intervals along the base of the drop to determine the thickness of the vapour layer.
The vapour layer was found to be 0.06mm for most of the length of the drop edge except in places where the edge curved upwards and the gap was 0.26mm.
The flow of vapour from the supporting layer considered as the escape of fluid from an orifice.
The rate of escape of a fluid from an orifice is governed by the geometrical shape of the orifice and the area of discharge. Again I am not including the calculations, evaporation rates and so on.
This method determined the vapour layer at the drop bottom edges to be 0.06mm
(Published values for the Coefficient of discharge (Cd) used were often for large hydraulic engineering projects. In order to check these applied to the very small scale Leidenfrost drops the following experiment was made:-
Air was released, through a rectangular slit of similar area (4 sq mm) and aspect ratio to that of a 2ml drop having a vapour layer thickness of 0.06mm, from a spherical balloon, to deliver air at constant pressure for a decreasing volume. Air pressure was measured with a U tube manometer and balloon volume determined from radius measured with external calipers as air was released over 5 second intervals.
The Cd values were in the same range as those used in the vapour layer measurements determined by flow from an orifice.
A Capacitance Method
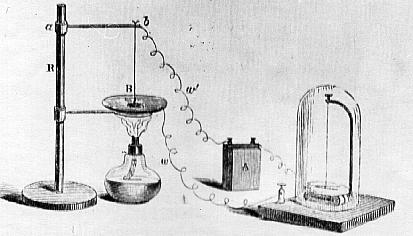
This is Tyndalls version of Poggendorf`s suggestion to demonstrate the existance of an insulating vapour layer. If the drop touches, or is made to touch the surface, the circuit is completed and the Galvanometer needle turns.
This gave me the idea to try and measure the capacitance of the capacitor made up of the drop and heated surface as plates and the vapour layer as dielectric.
For several approaches and other investigations e.g. volume to area changes due to evaporation, the drop area needed to be determined.
Measuring Drop Base Area: -
Sheets of glass had mm graph paper glued to one face. The opposite face was coated with vaseline renedered smooth in a flame. A drop was carefully set up on the greased surface and had the apearance of a Leidenfrost drop. The area was measured by the method of counting squares. For the drop volumes used - 2ml and less - the method proved okay.
Measuring Capacitance:-
Remember this investigation was carried out in the early 1970s. The only capacitance bridge was a valve version unable to go into low pf regions. So all capacitance had to be measured against a standard capacitor the value of which had then to be deducted from the measured value. (It may be of some historic interest to tell you that the digital calculator appeared - fixed decimal point version - at this time -oh to have had available all the marvellous digital meters we now have.)
With area known, capacitance measured and a dielectric value for air the only unknown was the plate separation distance = the vapour layer thickness. This varied all over the place and I did not find it came within acceptable ranges of that measured by the other methods. There are several reasons not least of which was the means of measuring capacitance! The whole vapour layer was involved not just that at the drop edge. The dielectric for the steam layer was assumed from dry air and now i feel it was probably wrong.
A Summary of Results
For drops of 3.5%NaCl and of volumes 1ml and less:-
Area of drop exposed to heated surface Ab = (190± 4)V sq m
Vapour layer pressure = 51.57 Pa
Vapour layer thickness at drop edge 0.06 mm
Vapour flow rate from drop edge Q = Cd Ao = sq root of (2(p1 - p2)/ r) ml per second
(Where Cd is co-efficient of discharge; Ao is the area of the annulus of the vapour escape - area of orifice; (p1-p2) is pressure excess in vapour layer = 51.57Pa; r is density of vapour (assumed to be that of air)
Area of orifice of escaping vapour Ao = 2p r d ........(r to be determined from Ab, d = 0.06 mm)
Total evaporation to related to drop volume t = (V/ 7.4 x 10¯9) raised to the 1/2.4 ....... seconds
Drop volume to total evaporation time (t) ....V = 7.4 x 10¯9 x t raised to the 2.4 power .... litres
Evaporation rate dV/dt = 1.68 t (to the 1.4) x 10¯5 ...... ml per second