
Colin Pounder Discovers Charged Solute Particles
from Leidenfrost Boiling
`Neque accendunt lucernam, et ponunt eam sub modio, sed super candelabrum, ut luceat omnibus qui in domo sunt.` Mt.5:15
I cast modesty to the winds and set up my light on a candlestick for this one! You know how you read of legendary scientific discoveries which seemed to come out of nowhere and suddenly, "Eureka!" well now you can share the experience for real with me.

Apparatus for measuring charge on a Leidenfrost drop. On the left is the electrometer - centre is a probe made from the body of a spark plug to withstand heat and remain insulated and connected to the meter via a screened coaxial connection - right is a dish on a hotplate. In front is the brass used for most of the investigation of drops.
During the later stages of investigating drop behaviour and shortly after devising this probe set up to measure capacitance I decided to examine drops to see if there was any electrical activity. One of the earlier investigators had stated that the Leidenfrost drops did not produce any electricity. (That was with water).
Recall how I wanted to extend the lifetime and in effect magnify the droplets of transition boiling which Blanchard had found produced charge which charged the clouds at Surtsey. So to do this I now used only saline solution drops (3.5%NaCl). This is the average salinity of the seas and oceans.
To measure out the correct volume I used either a pipette or calibrated syringes. Drops of solution behaved as did those of distilled water, forming rosettes sometimes, waves on the surface on occasions, or just remaining stationary.
With the apparatus shown above I found that saline solution drops produced electric charge but intermittently. A flick of the electrometer needle -back to zero - another flick and so on getting faster until the drop shrank and just a pellet of salt was left stuck to the probe.
I would let a drop diminish in size then add more solution. In this way I could feed a drop for many minutes and the remaining salt was a dome like that of a drop. I tend to observe for one particular item at a time. So once I had noted the electricity I wondered why it was generated in pulses and watched drop carefully for some change in behaviour.
I discovered that a saline solution drop would slowly diminish in size, as you would expect, when suddenly it went "Crack!" sometimes broke up and reformed or just sort of shook all over and settled down again. This was when electricity registered on the electrometer.
Goodness knows how many drops I watched and how many wonderful notions (the correct term is hypotheses) I had to explain this. For some reason I had the feeling that many of these drops had waves passing over the surface. Maybe if I could measure wavelength then I would find something.
I made a stroboscope from a disc of black card driven by a small model motor fed from a battery through a rheostat. With a photocell opposite a light and the disc with its slots interposed I could switch on and off a Dekatron timing unit and so determine the speed of the stroboscope.
As a light source for the stroboscope I used a slide projector. For hours I watched drops and waves but none ever strobed to a standstill. In the heat of that unventilated room and bent over the table staring at that flickering light my back ached intolerably. I gave up. Switched off the little motor and sat down in a chair with a great sigh of relief from backpain and thoughts of another night wasted, another brilliant (hypothesis) rubbished.
The last of the drops cruised around on the hot surface and went "Crack" a few times then left a bit of salt. It was then I noticed on the black card of the strobe curved streaks of white. The disc was about half a metre from the dish. I tasted the curved white streaks - they were salt. As I was wondering how the salt had got from the drop to the disc - remember my mind was set on something to do with waves on the drop surface - I stretched back in the chair and saw what looked like tiny brilliant white stars slowly coming down from the ceiling and lit up by the slide projector light.
I cannot describe what I felt, wonder, awe, a sense of the beauty of these tiny specks of light. Now I know I was as far as I am aware the first person ever to see the salt particles from salt water undergoing Leidenfrost boiling.
(Later Blanchard would confirm he and Woodcock had never seen particles. The salt on slides was in `mishappen lumps`.) I was the first to see the electrically charged salt particles from sea water contact with a heated surface -in Nature - lava.
We had the Total Eclipse of the Sun on Wednesday (11th August 1999) earlier this week. The feeling I am so glad so many people felt is similar to what I felt alone that night. For billions of years something has been happening and for that `one moment in annihilations waste` as an individual a person can say,"I experienced that for myself alone".
From this point on my whole research swung to study the particles. As Bill Bright told me - "Nature has granted you a pearl". For myself I feel it was a gift from God.
The work continued for another five and a half years. Leaving aside all the measuring, calculating and finally typing up and binding the thesis - I was determined the whole thing was to be my effort - hence why some of the photography here may be non-professional because I had to teach myself all the ins and outs of the subject - at the same time learning to one finger type - so please excuse all the errors you may find. Anyway here is a brief outline of the results:-
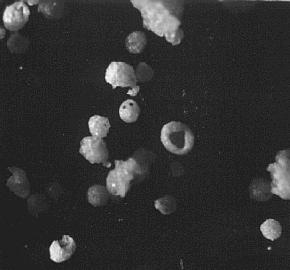
Salt specks from Leidenfrost boiling.
At first to get a look at the tiny specks of salt I listened from a drop to go Crack then waved a microscope slide about horiozontally and hoped for the best. This picture and the next show some results. I am sorry I cannot remember at what magnification these are.
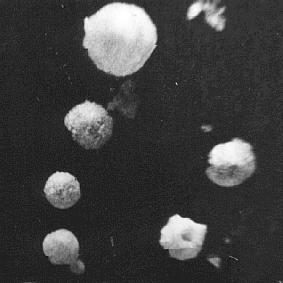
The specks are spherical and hollow
I surmised that the drop fired off droplets of solution and that as these floated in the air they evaporated off water. Their solution would become more concentrated and a skin of salt would form which trapped concentrated solution inside. Continuing evaporation through the skin made this rigid and slower crystallisation would continue inside the droplet/speck. Remaining solution coming under ever increasing pressure would burst out through the surface - making the small exit hole - and the speck/particle would dry out and float like a miniature geode charged with electricity.
To examine this idea I drew out glass to less than a human hair diameter - in a flame - and suspended a droplet of salt solution on it and fixed it under a microscope. I broke a torch bulb to make a small electric one bar electric fire which I fed from a battery via a rheostat. I brought the tiny glowing element near to the droplet and watched crystalline salt form on part of the surface and slowly spread to enshroud the droplet. After a while the salt coated droplet broke open and left a near spherical salt speck with an exit hole.
I also prepared bowls of saturated salt solution and examined the surface layers of crystalline material - fine on the surface where more rapid evaporation took place - large cubic crystals into the liquid under this surface.
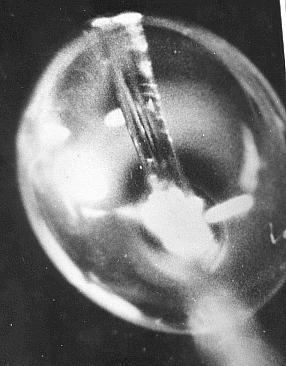
A droplet on glass drawn out to about the diameter of a hair.
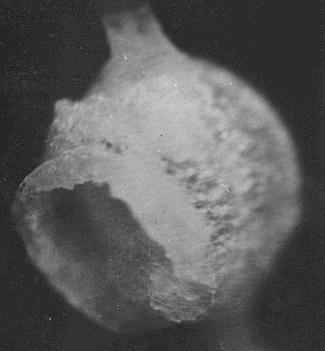
A Salt speck produced by heated element held near the above droplet.
I have the whole sequence of this but am too tired to search it all out and scan it -mea culpa!
The Salt Specks in glorious close up
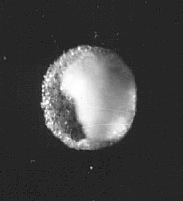
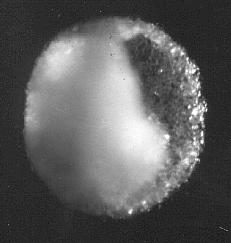
Salt specks from Leidenfrost boiling. 10 microns diameter photographed under an optical microscope. The surface is of fine crystalline salt the inside walls are covered with larger crystals which formed more slowly due to slowed evaporation. The opening is where solution was expelled by the inward acting pressure as internal crystals formed.
When the drop made a loud crack droplets were emitted from one place i.e. not all over the drop. With patience I caught droplets from such emissions on to slides blackened with candle smoke. I could then count the discs made by impacted droplets and get some idea of the number given off each time.
The probe used to detect and measure charge, via the electrometer, I now connected to a pulse counter. Each time droplets were emitted a pulse was registered. In this way I was able to gain some estimate of the number of droplet/speck/particle emission from drops of particular volumes.
The number of emissions from a drop of initial volume 1ml 3.5%NaCl solution ranged from 613 to 2,777. The average = 1,444.
From this it was possible to determine that from a 1 ml drop of 3.5%NaCl solution some 6 x 10 to the 5th particles are emitted.
Counts etc were all plotted as histograms and emissions to drop size, particles to drop size, duration of particle emissions , equations derived, etc etc were made.
Electrical
The average charge per particle emission ranged from 0.1 x 10¯10 Coulombs to 0.5 x 10¯10 C.
From which an average charge per particle of 10¯14C to 10¯13C with a net +.
This is very high . Usually in spraying, raindrop break up and suchlike the charges are in the 10¯19C and net -.
Dark Field Ultramicroscope
I constructed a dark field ultramicroscope. This consisted of an insulated case with two microscope slides - rendered conducting on one surface by a molecular layer of tin (I had to buy these which used up the cash for a month ) in parallel about ¼inch apart. The halogen projector shining straight in to the camera with a strobe disc interposed. A small black disk and various lenses gave a cone shaped shadow into the space between the parallel plates. The camera was focused an a weighted hair hung in the gap. The plates/conducting slides were connected to a high voltage battery pack. The depth of field was virtually zero.
Through the camera a black disc is seen surrounded by brilliant light. Any dust - Lycopodium pollen was used in trials - falling into the dark disc area lights up as a point of light. By projecting the negative on to a squared screen the rate of fall due to gravity may be calculated, any deflection towards a plate due to the particle carrying its own electrical charge may be measured. Hence the size and the charge of each individual particle may be determined.
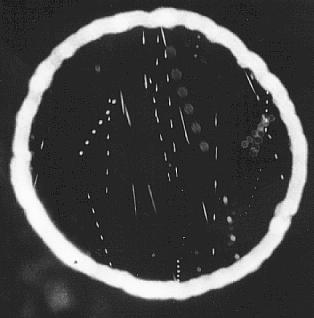
Salt specks from Leidenfrost boiling in the field of the ultramicroscope.
Dotted tracks are small sized particles, dashes are larger,moving more quickly, discs are out of focus and not used.
Three thousand slides were taken originally and the tracks measured by hand as described.
Other materials were used for the heated surface and other aqueous solutions - spring water -sea water (same as NaCl) - rainwater - water filtered after mixing with volcanis ash - pumice and powdered lava -were investigated - all produced electrically charged solute particles.
Summary of electrical properties of Leidenfrost drops.
Leidenfrost boiling of saline solution produces electricity.
A Leidenfrost drop has a net negative polarity, the opposite polarity of charge is assumed on the particulate cloud.
Average charge per particle all radii is 10¯14 Coulombs
Charge on 10 micron particles is 10¯14C to 10¯17C
Charge increases with particle radius.
Where the specks/particles come from
In other words particle emission from drops.
Briefly what happens is this: Evaporation/vapourisation is most from nearest to the hot surface.
As this takes place the drop becomes more and more saturated with salt (or other solute). For NaCl when the 3.5% concentration reaches about 36% solid solute crystallises on the surface. For most Leidenfrost drops this is on the underside. When this layer grows over a sufficient area the vapour support layer is shut off! The drop crashes on to the hot surface at several hundred degrees in tempereature.
At least two things happen - the crystalline salts decrepitates into charged fragments - some of the drop solution is flash boiled into steam. Both are directed by the `walls` of hot surface and drop above to hurtle outwards from beneath the drop and into the air/atmosphere -where the droplet and speck formation then take place.
The form of charging is tribo-electric - frictional electricity both in decrepitation and in the material sliding across the surfaces on its way out into the air where it forms highly charged particles.
To examine this Bill Bright arranged for me to use a high frame speed camera and here are some results from the film:-
Time between frames is 3 x
10¯3 seconds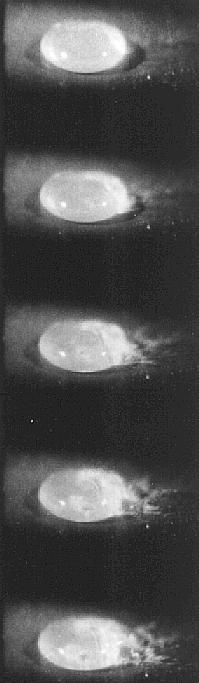
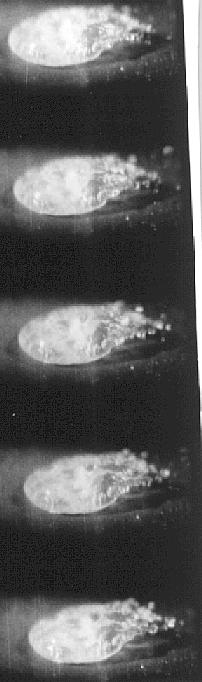
A Saline Solution drop Leidenfrost Boiling -emitting Triboelectrically charged material.
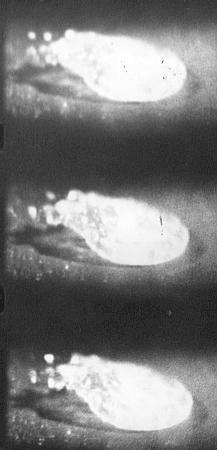
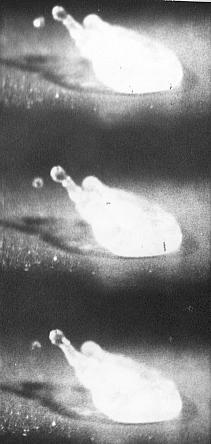
Another saline drop undergoing Leidenfrost Boiling and emitting electrically charged material which will form charged solute particles.
The high charge on each particle needs less of them to accumulate charge in a cloud.
Most volcanoes are phreatic which is to say they have water either in crater lakes, as junior water in the rocks of the magma chamber, as internal lakes in caverns (See Humboldt`s exploration of the Andes) and or in contact with the waters of the sea. At times of eruption the solution contact with hot lava produces billions of highly electrically charged particles into the clouds associated with the volcano. This electricity discharges as Volcanic Lightning.
There is reason to believe that the same process is also involved in earthquake lights. But that as they say is another story.